Product Description and Product Data
This is an all-inclusive cell-based luciferase reporter assay kit targeting the Human Mineralocorticoid Receptor (MR). INDIGO’s MR reporter assay utilizes proprietary mammalian cells that have been engineered to provide constitutive expression of the MR. In addition to MR Reporter Cells, this kit provides two optimized media for use during cell culture and in diluting the user’s test samples, a reference agonist, Luciferase Detection Reagent, and a cell culture-ready assay plate. The principal application of this assay is in the screening of test samples to quantify any functional activity, either agonist or antagonist, that they may exert against human MR. This kit provides researchers with clear, reproducible results, exceptional cell viability post-thaw, and consistent results lot to lot. Kits must be stored at -80C. Do not store in liquid nitrogen. Note: reporter cells cannot be refrozen or maintained in extended culture.
Features
Clear, Reproducible Results
- All-Inclusive Assay Systems
- Exceptional Cell Viability Post-Thaw
- Consistent Results Lot to Lot
Product Specifications
| Target Type | Nuclear Hormone Receptor | ||
| Species | Human | ||
| Receptor Form | Native | ||
| Assay Mode | Agonist, Antagonist | ||
| Kit Components |
| ||
| Shelf Life | 6 months | ||
| Orthologs Available | No | ||
| Shipping Requirements | Dry Ice | ||
| Storage temperature | -80C |
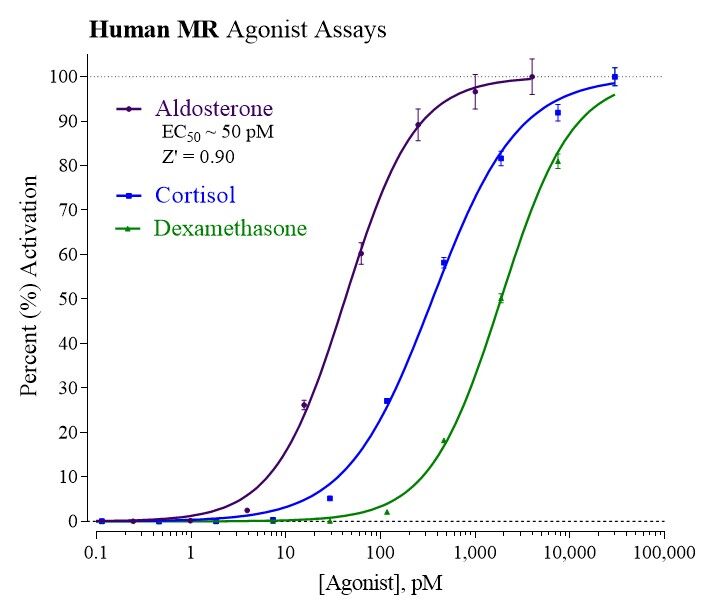
Data
Target Background
The mineralocorticoid receptor (MR), also called aldosterone receptor, is a receptor with high affinity for mineralocorticoids. It belongs to the steroid hormone receptor family where the ligand diffuses into cells, interacts with the receptor, and results in a signal transduction affecting specific gene expression in the nucleus. The gene for the NR3C2 (located on chromosome 4q31.1-31.2) encodes for the 107 kDa MR protein.
MR is expressed in many tissues, such as the kidney, colon, heart, central nervous system (hippocampus), brown adipose tissue, and sweat glands. In epithelial tissues, its activation leads to the expression of proteins regulating ionic and water transports (mainly the epithelial sodium channel or ENaC, Na+/K+ pump, serum and glucocorticoid induced kinase or SGK1) resulting in the reabsorption of sodium, and as a consequence an increase in extracellular volume, increase in blood pressure, and an excretion of potassium to maintain a normal salt concentration in the body.
The receptor is activated by mineralocorticoids such as aldosterone and deoxycorticosterone as well as glucocorticoids, like cortisol and cortison. It also responds to some progestins. Spironolactone and eplerenone are mineralocorticoid receptor antagonists. Activation of the mineralocorticoid receptor, upon the binding of its ligand aldosterone, results in its translocation to the cell nucleus, homodimerization and binding to hormone response elements present in the promoter of some genes. This results in the complex recruitment of the transcriptional machinery and the transcription into mRNA of the DNA sequence of the activated genes.
INDIGO’s Mineralocorticoid Receptor Reporter Assay Systems utilize proprietary mammalian cells engineered to express human NR3C2 protein, commonly referred to as MR.
The principle application of this assay product is in the screening of test samples to quantify functional activities, either agonist or antagonist, that they may exert against the human mineralocorticoid receptor.
Citations
Also available as a service