Drug Discovery Assay Kits: Comprehensive & Reliable Screening Solutions
Rapidly screen therapeutic candidates today and accelerate drug development decisions for a better tomorrow.
With INDIGO’s Drug Discovery Assay Kits, you can:

Showing all 3 results
-
Expression Profiling of Clinically Relevant CYPs Assay Kit
Species
Human
This Expression Profiling of Clinically Relevant CYPs assay kit contains optimized reagents for the culturing and treatment of upcyte® hepatocytes to assess drug-induced changes in CYP3A4, CYP1A1, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1.
The kit provides two aliquots of upcyte® hepatocytes, two cell culture-ready assay plates, optimized Cell Culture Medium for use in all steps of the assay procedure, and three reference compounds (rifampicin, β-naphthoflavone, and CDCA) that activate one or more of the primary xenobiotic-sensing receptors: PXR, CAR, AhR, and FXR. Upon activation, these nuclear receptors modulate the expression of the CYP genes. Also included are seven sets of validated qPCR primers for quantifying drug-induced changes in the expression of CYP3A4, CYP1A1, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1, as well as primers for ACTB.
Please note: This kit does not include reagents or protocols for cell lysis, RNA isolation, cDNA preparation, or qPCR assays.
-
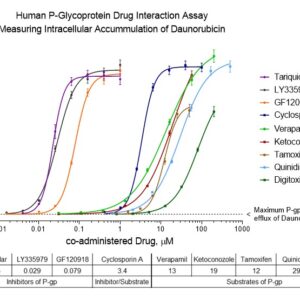
Human P-Glycoprotein / MDR1 Drug Interaction Assay
Species
Human
The primary application of INDIGO Biosciences MDR1 drug interaction assay kit is to rapidly assess drug candidates as either inhibitors, substrates, or non-substrates of P-glycoprotein. This is accomplished by co-treating the cells with varying concentrations of the test drug and a fixed concentration of daunorubicin, a well-characterized fluorescent substrate for P-glycoprotein mediated transport. HCT-Pgp cells are a proprietary cell line that have been extensively selected for high-level expression of native P-glycoprotein.
The kit contains two aliquots of HCT-Pgp cells and two black, sterile, collagen-coated 96-well assay plates. In addition to HCT-Pgp cells and assay plates, this kit includes Cell Recovery Medium (CRM-p) used to perform a rapid thaw of the -80°C HCT-Pgp cells, Compound Screening Medium supplemented with daunorubicin (CSM+DR) used to prepare drug treatment media, Wash Buffer, two aliquots of Lysis Reagent, and the potent reference inhibitor Tariquidar.
-
in vitro Screening for Drug-Induced Hepatotoxicity Assay Kit
Species
Human
The principal application of this assay is to rapidly screen test compounds to identify those that induce acute liver cell toxicity.
This kit includes two aliquots of cryopreserved Luminescent Reporter Hepatocytes (upcyte®), donor 10-13, isolated from an adult Caucasian female, that have been further modified to constitutively express the luciferase enzyme. In addition to two aliquots of the reporter hepatocytes, the kit provides two cell culture-ready assay plates, optimized Cell Culture Media (CCM) for use in all steps of the assay procedure (cell thawing, seeding, and preparation of the treatment media), luciferase detection reagent, and a reference compound that provides a positive control for hepatotoxicity.
The reagents and materials provided in this assay kit are formatted to allow the user to choose between two alternative assay setups. In one scenario 48 culture wells may be setup at two different times. In the other assay scenario 96 culture wells may be setup at one time.

Evaluate Drug Candidates’ Safety from Every Angle with INDIGO’s Drug Discovery Assays
Did you know that almost 90% of candidate drugs fail in the first stage of trials?
Your drug lead can reverse the trend!
With INDIGO’s in vitro drug discovery assays, save resources and energy by identifying and characterizing adverse effects and potential risks before your drug candidate progresses further in the drug development process.
Explore INDIGO’s in vitro solutions in the broad categories of Prospective Screens, Safety Pharmacology, and Retrospective Screens, and start focusing your efforts on the best drug leads only.

Prospective Screens
Explore drug-drug interactions with INDIGO’s Nuclear Receptor Profiling and accurately predict the adverse effects of your molecule of interest.
Or dig deeper downstream of nuclear receptor activation through quantification of gene expression using INDIGO’s assay services.
Safety Pharmacology
Evaluate your compounds on major receptors with INDIGO’s reporter assay panels to understand their biological and toxicological effects better.
Identify therapeutic agents with the highest selectivity and lowest potential for off-target and unwanted effects with INDIGO’s cell-based receptor assays.

Retrospective Screens
Unexpected results from clinical trials or animal studies? Trouble-shoot with INDIGO's large portfolio of ortholog assays to quickly determine if the response observed in laboratory animals is concordant with humans.
Explore Additional INDIGO’s in vitro Solutions to Improve Drug Discovery
Quickly gain insights into the mechanisms underlying the involvement of key receptors in various diseases with INDIGO’s reporter assays.
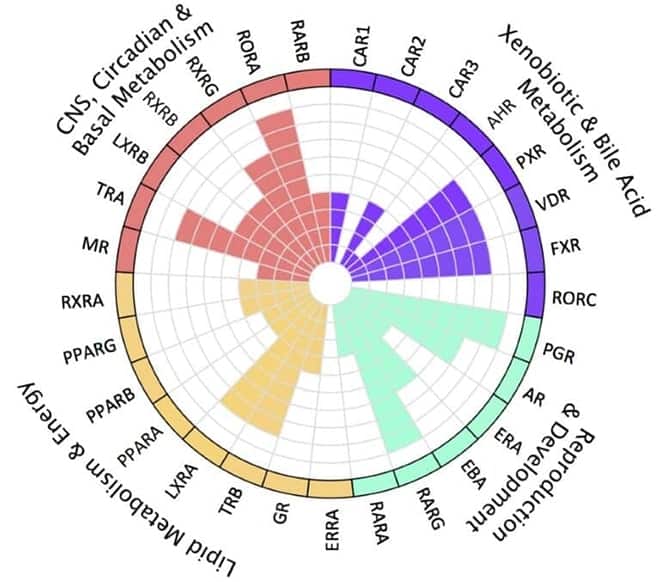
Understand a compound's on- and off-target effects and liabilities on overlapping pathways with nuclear receptors functional classifications.
Frequently Asked Questions
Each kit includes luciferase reporter cells, an assay plate, all required reagents (optimized culture media, positive-control agonist, and detection reagent), and a detailed Technical Manual. A luminometer is required for Relative Light Unit (RLU) measurements.
INDIGO's Nuclear Receptor Assay Kits use transiently transfected cells, less prone to genetic drift and batch-to-batch variations. Moreover, they do not require cell culture, significantly reducing time and contamination risks. Simply thaw the reporter cells, and you are ready to perform the assay.
INDIGO’s Nuclear Receptor Assay Kits are not for diagnostic purposes.
We do not recommend normalizing RLU data to cell numbers or protein content. The suspension of reporter cells is a ‘master reagent’ that should be dispensed with high precision into all assay plate wells. Any significant downstream variability in cell number or health will result from cytotoxicity induced by the test compound treatments. To identify and weed out false-positive data, it is essential to perform cytotoxicity analyses for antagonist-mode assays (and inverse-agonist mode assays). INDIGO’s Live Cell Multiplex (LCM) Assay works well for that purpose. However, attempting to normalize RLU data from treated cells in metabolic crisis to untreated, healthy control cells can result in gross misinterpretations of the data when performing cell-based trans-activation assays (which rely on the coordinated cell-cycle processes of gene induction, transcription, translation, mRNA and protein turn-over) “healthy cells” and “dying cells” are not equal units, so attempting to normalize assay data to any cell-related endpoint is not a sound method.
The concentration of organic solvent carried over into the assay wells should not exceed 0.1%. We recommend no more than 0.4% DMSO carry-over into the assay wells.