Toxicology Assay Kits
Rapidly assess therapeutic candidates’ potential for drug-drug interactions and accurately predict xenobiotic-induced liver toxicity. INDIGO’s toxicology assay kits provide rapid, reliable results for:

Showing all 3 results
-
Expression Profiling of Clinically Relevant CYPs Assay Kit
Species
Human
This Expression Profiling of Clinically Relevant CYPs assay kit contains optimized reagents for the culturing and treatment of upcyte® hepatocytes to assess drug-induced changes in CYP3A4, CYP1A1, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1.
The kit provides two aliquots of upcyte® hepatocytes, two cell culture-ready assay plates, optimized Cell Culture Medium for use in all steps of the assay procedure, and three reference compounds (rifampicin, β-naphthoflavone, and CDCA) that activate one or more of the primary xenobiotic-sensing receptors: PXR, CAR, AhR, and FXR. Upon activation, these nuclear receptors modulate the expression of the CYP genes. Also included are seven sets of validated qPCR primers for quantifying drug-induced changes in the expression of CYP3A4, CYP1A1, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2E1, as well as primers for ACTB.
Please note: This kit does not include reagents or protocols for cell lysis, RNA isolation, cDNA preparation, or qPCR assays.
-
Human P-Glycoprotein / MDR1 Drug Interaction Assay
Species
Human
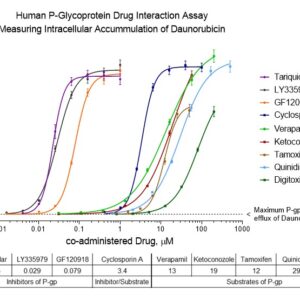
The primary application of INDIGO Biosciences MDR1 drug interaction assay kit is to rapidly assess drug candidates as either inhibitors, substrates, or non-substrates of P-glycoprotein. This is accomplished by co-treating the cells with varying concentrations of the test drug and a fixed concentration of daunorubicin, a well-characterized fluorescent substrate for P-glycoprotein mediated transport. HCT-Pgp cells are a proprietary cell line that have been extensively selected for high-level expression of native P-glycoprotein.
The kit contains two aliquots of HCT-Pgp cells and two black, sterile, collagen-coated 96-well assay plates. In addition to HCT-Pgp cells and assay plates, this kit includes Cell Recovery Medium (CRM-p) used to perform a rapid thaw of the -80°C HCT-Pgp cells, Compound Screening Medium supplemented with daunorubicin (CSM+DR) used to prepare drug treatment media, Wash Buffer, two aliquots of Lysis Reagent, and the potent reference inhibitor Tariquidar.
-
in vitro Screening for Drug-Induced Hepatotoxicity Assay Kit
Species
Human
The principal application of this assay is to rapidly screen test compounds to identify those that induce acute liver cell toxicity.
This kit includes two aliquots of cryopreserved Luminescent Reporter Hepatocytes (upcyte®), donor 10-13, isolated from an adult Caucasian female, that have been further modified to constitutively express the luciferase enzyme. In addition to two aliquots of the reporter hepatocytes, the kit provides two cell culture-ready assay plates, optimized Cell Culture Media (CCM) for use in all steps of the assay procedure (cell thawing, seeding, and preparation of the treatment media), luciferase detection reagent, and a reference compound that provides a positive control for hepatotoxicity.
The reagents and materials provided in this assay kit are formatted to allow the user to choose between two alternative assay setups. In one scenario 48 culture wells may be setup at two different times. In the other assay scenario 96 culture wells may be setup at one time.